Abstract
Introduction
Acute myeloid leukemia (AML) is primarily a disease of older adults, with 40-50% of patients ≥ 70 years old at diagnosis. The optimal selection of frontline therapy remains controversial, with intensive chemotherapy (IC) often favored in AML patients who present with a proliferative phenotype (P-AML) compared to a non-proliferative phenotype (NP-AML), yet evidence to support this approach is lacking. We aimed to investigate the outcomes of older AML patients based on treatment choices including intensive chemotherapy (IC), hypomethylating agents (HMA) and low intensity therapy (LI) (excluding HMAs), stratified on the basis of a proliferative and non-proliferative phenotype.
Methods
We retrospectively analyzed clinical and laboratory parameters along with treatment outcomes in 983 elderly AML patients treated at Moffitt Cancer Center between 1995 and 2016. Patients with prior exposure to hypomethylating agents for non-AML diagnoses were excluded. Patients were placed into one of four cohorts based on the type of frontline therapy received: Intensive chemotherapy (IC) (defined as daunorubicin/cytarabine or equivalent); hypomethylating agents (HMA) (azacitadine (AZA) or decitabine (DEC)); low intensity therapy (LI) (defined as low-dose cytarabine or similar agent excluding HMAs); or supportive care (SC) (including hydroxyurea). P-AML was defined as presence of ≥30% bone marrow (BM) myeloblasts, whereas NP-AML was defined as 20%-29% BM myeloblasts at diagnosis. Age, type of AML, history of antecedent hematologic disease, cytogenetics, ECOG performance status, Charlson comorbidity Index, complete blood counts and myeloblast percentage at diagnosis were compared for each patient. Kaplan-Meier analysis was performed to calculate overall survival and the log-rank test was used to determine significance where a p-value of <0.05 was defined as significant.
Results
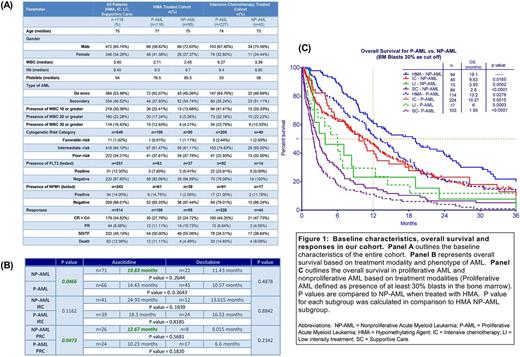
Of the 983 patients, 718 patients had data regarding BM blast percentage at diagnosis without previous exposure to HMA who were included in the analyses. Of these, 479 (66.71%) were classified as P-AML and 239 (33.29%) as NP-AML. Baseline characteristics are described in Figure 1A. Overall survival (OS) of the entire cohort was 8.0 months (mo). No significant difference was noted in OS between NP-AML and P-AML (8.9 vs. 7.7mo respectively, p=0.1741). The analysis based on treatment modality with focus on HMA and IC revealed that HMA therapy in NP-AML produced a significantly superior OS compared to IC (19.1 vs. 9.6 mo, p=0.0165), whereas no significant OS difference was found in the P-AML patients (13.3 (HMA) vs. 10.3 mo (IC), p=0.3135). In the HMA cohort, OS was better in NP-AML compared to P-AML (19.1 mo vs. 13.2 mo, p=0.0276). Survival with LI treatment was inferior to IC or HMA in both P-AML and NP-AML cohorts (Figure 1C).
The HMA treated cohort was then analyzed, based upon risk stratification using NCCN cytogenetic risk categories. Sample size was too small to assess OS in the favorable-risk patients (NP-AML, n=1; P-AML, n=1). In the intermediate risk patients (IRC), OS was similar between NP-AML and P-AML (23.97 months vs. 17.5 months, p=0.1650). In the poor-risk cytogenetics (PRC) subgroup, survival was superior in the NP-AML cohort compared to P-AML (11.58 months vs. 9.37 months, p=0.0132).
Finally, outcome based upon the specific HMA administered (AZA vs. DEC) was compared (Figure 1B). AZA treatment was associated with a trend favoring improved OS in NP-AML compared to DEC (19.83 months vs. 11.13 months, p=0.2644). Moreover, AZA treatment statistically improved OS in NP-AML compared to P-AML (19.83 months vs. 14.43 months, p=0.0466). Survival outcomes with AZA vs DEC based on cytogenetic risk categories are illustrated in Figure 1B.
Conclusions
HMAs represent effective frontline therapy for elderly patients with P-AML or NP-AML, with particular benefit compared to IC or LI in the NP-AML subset. In patients harboring poor-risk cytogenetics, HMA therapy produced an improvement in OS in NP-AMLvs.P-AML.
Sallman: Celgene: Research Funding. Padron: Incyte: Honoraria, Research Funding. Komrokji: Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria. Sweet: Incyte: Research Funding; Ariad: Consultancy, Speakers Bureau; Karyopharm: Consultancy, Research Funding; Pfizer: Consultancy; Otsuka: Consultancy; Novartis Pharmaceuticals: Consultancy, Speakers Bureau. Lancet: Janssen: Consultancy; Erytech: Consultancy; BioSight: Consultancy; Bio-Path Holdings: Consultancy; Pfizer: Other: Institutional research funding, Research Funding; Jazz Pharmaceuticals: Consultancy; Celgene: Consultancy; Boehringer Ingelheim: Consultancy; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal